Overview of Commercial Water Softeners
Commercial water softeners are systems designed to remove hardness minerals, primarily calcium and magnesium, from water to prevent scale buildup, improve appliance longevity, and enhance cleaning efficiency in settings like hotels, restaurants, and industrial facilities. The choice of technology depends on the scale of operation, water quality, and cost considerations.
Main Technologies and Their Efficiencies
- Ion Exchange Softeners: These systems use resin beads to exchange hardness ions with sodium, offering high removal efficiency. They are ideal for smaller to medium-sized commercial applications due to ease of installation and maintenance.
- Lime Softening: This method adds lime and sometimes soda ash to precipitate hardness minerals, suitable for large-scale operations but less efficient in complete hardness removal compared to ion exchange.
- Nanofiltration: While less common, it can soften water by removing hardness minerals while allowing some dissolved solids to pass, often used in specific industrial applications.
Each technology has trade-offs in terms of efficiency, cost, and environmental impact, which are detailed below in the survey section.
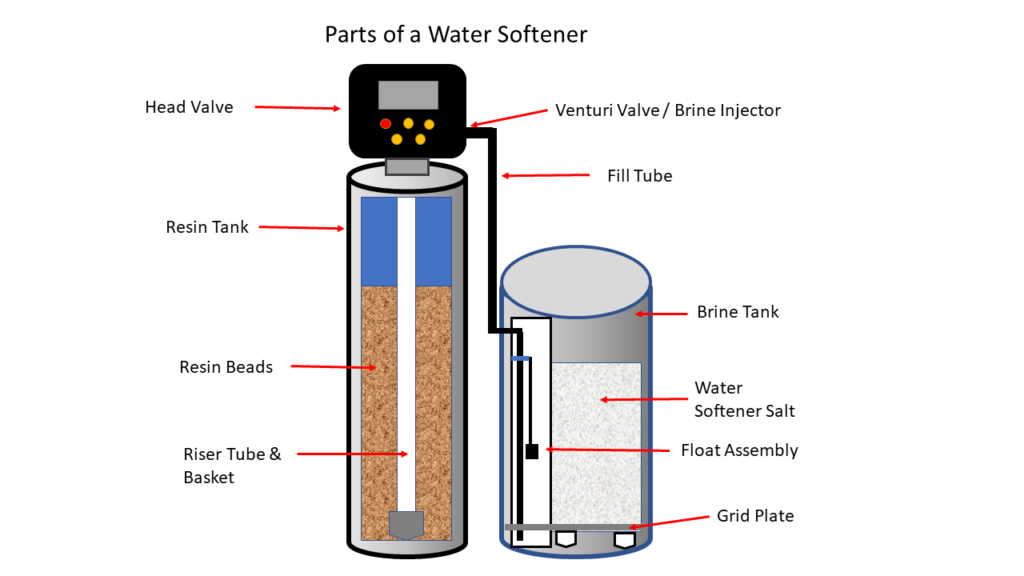
Survey Note: Comprehensive Analysis of Commercial Water Softener Technologies and Efficiencies
This note provides an in-depth examination of commercial water softeners, focusing on the technologies used—ion exchange, lime softening, and nanofiltration—and their respective efficiencies. It aims to cover all aspects relevant to commercial applications, including removal efficiency, operational costs, environmental impact, and suitability for different scales, based on recent research and industry practices as of February 28, 2025.
Introduction to Water Softening in Commercial Settings
Water softening is critical in commercial environments to mitigate the effects of hard water, which contains high levels of calcium and magnesium, leading to scale buildup in pipes, reduced efficiency of water-based appliances, and increased cleaning costs. Commercial water softeners are designed for high-volume usage, such as in hotels, restaurants, car washes, and industrial processes, where water quality directly impacts operational efficiency and equipment longevity.
Primary Technologies for Commercial Water Softeners
Ion Exchange Water Softeners
- Technology Description: Ion exchange softeners operate by passing hard water through a resin bed loaded with sodium ions. The resin exchanges calcium and magnesium ions with sodium, effectively removing hardness. The resin is regenerated using a brine solution (sodium chloride) to restore its capacity.
- Efficiency Metrics:
- Removal Efficiency: Research suggests ion exchange can remove up to 99% of hardness minerals, making it highly effective for achieving very soft water. For instance, studies indicate it can reduce hardness to less than 1 mg/L as CaCO3 in optimal conditions Ion Exchange & Water Demineralization Handbook | Veolia.
- Salt Efficiency: The efficiency is measured in grains of hardness removed per pound of salt, typically ranging from 3,000 to 15,000 grains per pound, depending on system design and operation What is the Salt Efficiency of an Ion Exchange Water Softener? | SoftPro® Water Systems News blog.
- Water Usage: Regeneration can use 25 gallons or more per cycle, but advancements like counter-current regeneration can reduce water and salt usage by up to 75% and 65%, respectively The Ultimate FAQ Guide:Ion Exchange | Water Softener System.
- Suitability: Ideal for smaller to medium-sized commercial applications due to lower initial capital costs and ease of maintenance. Automated regeneration cycles make it user-friendly for businesses like laundromats and restaurants.
- Operational Costs: Lower initial costs but higher ongoing expenses due to salt purchases and brine disposal. The cost per unit of hardness removed depends on salt efficiency, which can be optimized with modern controllers like Culligan’s GBE Electronic Controller, reducing salt consumption by at least 25% Industrial and Commercial Water Softeners – Culligan Industrial Water.
- Environmental Impact: Produces brine waste, which requires proper disposal to prevent environmental contamination, especially in areas with strict wastewater regulations.
Lime Softening
- Technology Description: Lime softening involves adding lime (calcium hydroxide) and sometimes soda ash (sodium carbonate) to raise the pH, causing hardness minerals to precipitate as calcium carbonate and magnesium hydroxide. These precipitates are removed through sedimentation and filtration.
- Efficiency Metrics:
- Removal Efficiency: Typically removes 80-90% of hardness minerals, with residual hardness often remaining at 50-85 mg/L as CaCO3, which can be desirable to prevent corrosion Lime Soda Ash Softening – Water.mecc.edu. For example, in cold lime treatment, calcium might be reduced to 35 ppm and magnesium to 70 ppm, depending on initial concentrations Cold Lime Softening – Clark’s Process – Definition | AWC.
- Chemical Usage: The amount of lime needed varies with water chemistry, typically requiring 1-2 parts lime per part hardness removed, but exact figures depend on alkalinity and other factors.
- Energy Consumption: Higher due to mixing, sedimentation, and filtration processes, especially in warm lime softening, which enhances efficiency by reducing solubility at higher temperatures The History of Water Treatment Science – Association of Water Technologies.
- Suitability: Best suited for large-scale municipal or industrial applications, such as water treatment plants, due to its ability to handle high volumes and additional benefits like silica and alkalinity reduction.
- Operational Costs: Higher initial capital costs for equipment like clarifiers and mixers, but potentially lower per-unit costs for high-volume operations. Requires trained staff for operation, adding to labor costs Comparison: Industrial Water & Municipal Water Softening – Cation Ion Exchange Water Softening vs. Lime Softening | Industrial Water Solutions.
- Environmental Impact: Produces sludge, which needs handling and disposal, posing environmental challenges, especially in areas with limited landfill capacity.
Nanofiltration
- Technology Description: Nanofiltration uses semi-permeable membranes to remove hardness minerals (divalent ions like calcium and magnesium) while allowing monovalent ions to pass, operating at lower pressures than reverse osmosis (RO).
- Efficiency Metrics:
- Removal Efficiency: Can achieve over 90% hardness rejection, but less effective for monovalent ions, making it a “softening membrane” rather than a complete demineralization solution Nanofiltration Membrane Softening – AMTA. For instance, it might reject 80% of salts but maintain higher TDS compared to RO.
- Energy Consumption: Operates at 50-150 psi, lower than RO (150-300 psi), but still more energy-intensive than ion exchange Advantages of Nanofiltration | American Water Chemicals.
- Suitability: Used in specific commercial applications, such as food processing (e.g., dairy, juice production), where partial demineralization is needed, and in areas with low TDS water Nanofiltration | Water Solutions – DuPont.
- Operational Costs: Higher capital and maintenance costs due to membrane replacement and pressure requirements, making it less common for general commercial water softening.
- Environmental Impact: Produces concentrate waste, similar to RO, requiring disposal, but less waste compared to lime softening sludge.
Comparative Analysis
To facilitate comparison, the following table summarizes key metrics for each technology:
| Technology | Removal Efficiency | Operational Cost | Suitability | Environmental Impact |
|---|---|---|---|---|
| Ion Exchange | Up to 99% | Moderate, salt-based | Small to medium commercial | Brine waste, disposal needed |
| Lime Softening | 80-90% | High initial, lower per unit for large scale | Large-scale municipal/industrial | Sludge production, disposal challenge |
| Nanofiltration | Over 90% hardness, partial TDS | High, membrane-based | Specific industrial applications | Concentrate waste, disposal needed |
- Removal Efficiency: Ion exchange leads with nearly complete hardness removal, followed by nanofiltration and lime softening, which leaves residual hardness.
- Cost Considerations: Ion exchange has lower initial costs but ongoing salt expenses, while lime softening’s high setup costs may be offset by economies of scale. Nanofiltration is costlier due to membrane technology.
- Environmental Impact: All technologies produce waste, with ion exchange’s brine and lime softening’s sludge requiring careful management, and nanofiltration’s concentrate adding to disposal challenges.
Emerging Technologies and Future Trends
While ion exchange and lime softening dominate, emerging technologies like electrochemical water softening are gaining interest. Electrochemical methods, such as cathode precipitation and homogeneous crystallization, aim to reduce chemical usage and waste, but as of February 28, 2025, they are still in research phases and not widely implemented in commercial settings Electrochemical water softening technology: From fundamental research to practical application – ScienceDirect. Salt-free options, like template-assisted crystallization (TAC), are also emerging for residential use but less common commercially due to lower effectiveness compared to ion exchange Drinking Water Treatment: Salt-Free Water ‘Softener’ Options – UNL Extension.
Practical Considerations for Commercial Applications
- For businesses with moderate water usage, such as hotels or restaurants, ion exchange softeners offer a balance of efficiency and cost, with modern systems incorporating brine reclamation to reduce salt and water usage by up to 33% Commercial Water Softeners – Water Control Corporation.
- Large industrial operations, like power plants, may prefer lime softening for its ability to handle high volumes and additional impurity removal, despite higher setup costs.
- Nanofiltration might be considered for niche applications, such as food processing, where partial softening and specific ion removal are required, but it’s less common due to cost.
Conclusion
In conclusion, ion exchange and lime softening are the primary technologies for commercial water softeners, with ion exchange being more efficient for smaller to medium-sized operations and lime softening for large-scale applications. Nanofiltration serves specific industrial needs but is less prevalent. The choice depends on removal efficiency, cost, waste management, and scale, with ongoing advancements aiming to improve efficiency and reduce environmental impact.


